This article is part of our special May 2020 issue. Download the full issue here.
Relation
Catinean A, Neag AM, Nita A, Buzea M, Buzoianu AD.bacillusspp. Spores – a promising treatment option for patients with irritable bowel syndrome.Nutrients. 2019;11(9):1968.
Objective
The aim of this study was to compare rifaximin followed by a nutraceutical or low fermentable oligosaccharide, disaccharide, monosaccharide and polyol (FODMAP) diet with spore-based probiotic (MegaSporeBiotic) therapy alone in patients with irritable bowel syndrome (IBS) without constipation.
Draft
A non-blinded, prospective, randomized, controlled clinical trial. Participants were randomized into 3 groups:
- G1, bei dem die Teilnehmer eine 10-tägige Kur mit Rifaximin (1.200 mg) erhielten, gefolgt von einer 24-tägigen Kur mit einem nutrazeutischen Inhaltsstoff Bifidobacterium longum W11, lösliche Ballaststoffe und Vitamine B1B2B6und B12.
- G2, bei dem die Teilnehmer einen 34-tägigen Kurs erhielten Bazillus spp probiotisch (Bacillus licheniformis, Bacillus indicus HU36™, Bacillus subtilis HU58™, Bacillus clausii, Bacillus coagulansalle von der Marke MegaSporeBiotic).
- G3, bei dem die Teilnehmer eine 10-tägige Behandlung mit Rifaximin (1.200 mg) erhielten, gefolgt von einer 24-tägigen Low-FODMAP-Diät.
Researchers obtained outcome measures at baseline, day 10 (for groups G1 and G3), day 34, and day 60.
Participant
This study included 90 patients with irritable bowel syndrome without constipation based on the Rome III criteria. Patients were between 18 and 75 years old and had a normal colonoscopy in the last 5 years, blood values within reference values and normal fecal calprotectin. Patients with documented food allergies, gluten intolerance or celiac disease, diabetes, thyroid disease, inflammatory bowel disease or other organic diseases, eating disorders (anorexia or bulimia), probiotics 1 month before the study, antibiotic treatment in the last 6 months, or specific diets were excluded.
Study parameters assessed
Researchers evaluated patients based on the IBS severity score (IBS-SS), quality of life for IBS patients (IBS-QL), and a rectal volume sensation test.
Key insights
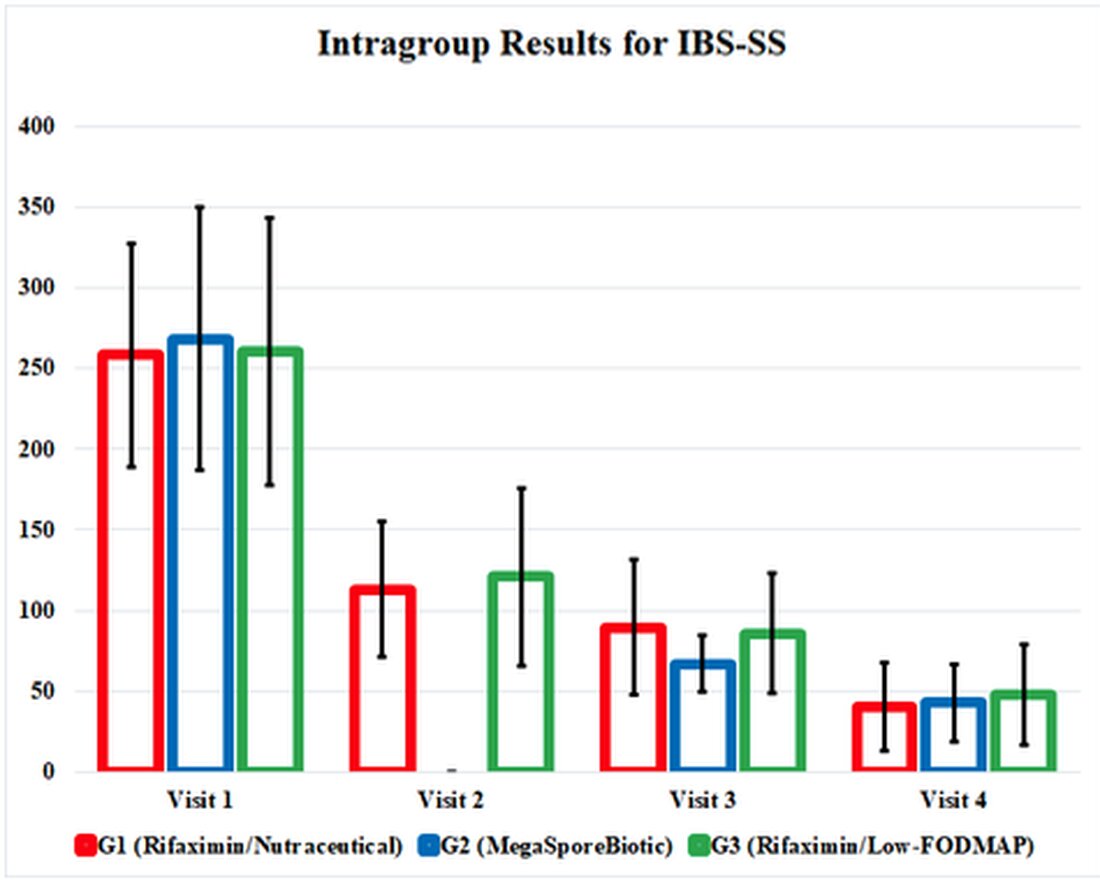
IBS-SS improved on every outcome measure for G1, G2 and G3 and, interestingly, improved equally by the end of the study. The MegaSporeBiotic group, G2, had earlier symptom improvement at Visit 3 (Day 34). Quality of life scores and rectal volume sensation test also improved in each group, with similar results in each group.
Practice implications
Irritable bowel syndrome is a common disorder affecting approximately 10% of the population, with significant gaps in reliable and cost-effective treatment strategies.1Our understanding of pathophysiology is rapidly expanding using systemic biology. The currently proposed model is a complex web of gut microbiota dysfunction, altered gut permeability, altered motility, gastrointestinal (GI) immune cell activation, visceral hypersensitivity, and abnormal gut-brain interactions.2Rifaximin first emerged as an effective treatment option for altering the GI microbiota in 2011 with the TARGET trial, which ultimately led to FDA approval of rifaximin for the treatment of IBS with diarrhea in 2015.3This study aims to show that non-antibiotic therapy by altering the microbiome with a spore-based probiotic may also be effective for the treatment of IBS with diarrhea (IBS-D).
One of the most exciting aspects of this study is the cost-effectiveness of spore-based probiotics compared to rifaximin.
This study contains several significant limitations, many of which the authors acknowledge. These include a lack of blinding and placebo, moderate rather than severe symptoms at baseline, a lack of breath testing for small intestinal bacterial overgrowth (SIBO), and the use of Rome III rather than Rome IV criteria. The researchers used Rome III because this study began before Rome IV, and the authors note that 90% of participants also met the new criteria.
There are additional limitations not discussed by the authors. The first and perhaps most important is that the rifaximin treatment group was undertreated in both dose and duration. The currently generally accepted dosage of rifaximin for IBS-D is 550 mg 3 times daily (1,650 mg total daily dose) for 14 days.4In this study, a total of 1,200 mg per day was used for 10 days, which is 52% of the effective dose. This leads to a significant result bias compared to the spore-based probiotic intervention.
Improved outcome measures could strengthen this study. First, the authors used a rectal volume sensation test at baseline and at each visit. This test is invasive, inconvenient, and has little support in the literature as an outcome measure for IBS-D.5Instead of the rectal volume sensation test, a non-invasive 3-hour lactulose breath test would improve the study design. This would have allowed the authors to include only patients with SIBO or to stratify responders in each treatment group based on SIBO status. Rezaie et al. found that patients with a positive lactulose breath test for SIBO responded significantly better to rifaximin therapy than IBS-D patients with a normal breath test.6Inclusion of a 3-hour lactulose breath test in this study would have clarified which patients were the best candidates for spore-based probiotic treatment versus rifaximin therapy.
The use of 2 treatment groups (rifaximin/low-FODMAP diet versus spore-based probiotic) or 2 treatment groups and a placebo group would have improved the clarity of the results. Blinding could eliminate another source of bias; However, it is impossible to blind therapeutic nutrition to usual nutrition and this is an ongoing challenge in nutritional research.
The effects of the therapeutic interventions in this study were buried in confusing and cumbersome tables, so I have highlighted positive results in a graphic illustrating the study's results. This graphic presents the study results and shows us how effective these therapies are compared to each other. It also highlights the fact that all 3 treatment groups continued to improve even after interventions were discontinued at Visit 3 (Day 34).
One of the most exciting aspects of this study is the cost-effectiveness of spore-based probiotics compared to rifaximin. The Rifaximin dose used in this study costs approximately $1,300, and the recommended dose is closer to $2,000. Insurance coverage often requires multiple medication failures and prior authorizations if treatment is covered at all. The dose and duration of the spore-based probiotic used in this study retails for $55. This represents a significant advantage of spore-based probiotic therapy. The authors did not report any side effects or participant dropouts, so it is difficult to factor these factors into a cost-effectiveness ratio.
It should also be noted that results were similar for spore-based probiotic therapy compared to the low-FODMAP diet treatment group. The low-FODMAP diet has been shown to be an effective nutritional strategy for treating IBS-D.7Although it is exciting to have an effective nutritional tool for IBS, this diet is very restrictive and has far-reaching psychosocial and nutritional effects.8In my experience, the low FODMAP diet is a more stressful diet compared to other, perhaps even more restrictive diets because the food choices are not intuitive. Patients must constantly use handouts and apps and therefore must become very vigilant to successfully follow this diet. In this regard, spore-based probiotic therapy offers a significant advantage.
Conclusion
Although this study has several methodological issues that introduce bias, it is important to recognize that the results of this study improve our understanding of treatment options for IBS-D. Spore-based probiotic therapy as a stand-alone therapy was associated with improvements in IBS severity, quality of life, and rectal volume sensation equivalent to those of rifaximin therapy followed by a low-FODMAP diet or probiotic therapy. Symptoms continued to improve for all 3 interventions after discontinuation of treatment, as evidenced by improved IBS severity scores at day 60 compared to day 34 after discontinuation of treatment. The most significant error in the study was the insufficient dose of rifaximin for treatment. Nevertheless, spore-based probiotic therapy offers a much simpler treatment at less than 5% of the cost of rifaximin. More research is needed before spore-based probiotic therapy can be claimed to be as effective as rifaximin, but it can certainly be considered when selecting treatment options for IBS-D patients.

 Suche
Suche
 Mein Konto
Mein Konto